2015年12月,董云伟教授课题组在《Functional Ecology》发表题为“Ecological relevance of energy metabolism: transcriptional responses in energy sensing and expenditure to thermal and osmotic stresses in an intertidal limpet”的研究论文,阐述了多环境因子相互作用对潮间带生物细胞能量状态的影响及其生态复杂性。
在自然环境中,生物体会在同一时间内受到多种环境因子的胁迫,这些因子的相互作用会对机体功能产生巨大的影响。而细胞的能量状态以及代谢调节对胁迫的响应是非常重要的,然而潮间带生物在应对多种亚致死环境胁迫时能量代谢响应仍不清楚。
本研究通过模拟了自然环境中多种环境因子对生物的交互作用,以帽贝(Cellana toreuma)为研究对象,研究了在高温、降水和干燥等条件下帽贝细胞内代谢调节和分解代谢相关基因的表达水平的变化。研究结果发现,热休克蛋白(hsp70),axin(Fu gene inhibition axis formation),代谢感受因子ampk(腺苷酸活化蛋白激酶)和sirt(沉默信息调节因子2相关酶1,5)以及代谢酶己糖激酶(hexokinase),丙酮酸激酶(pyruvate kinase),异柠檬酸脱氢酶(isocitrate dehydrogenase,idh)是非常合适的指示标记,通过其转录表达水平上的测定,可用来阐述嫁虫戚面对高温、干燥以及雨水刺激下的细胞能量的生理反应状态。
从基因表达模式以及频数分布图来看,高温、干燥、雨水单一胁迫或者多重环境因子的胁迫都可以引起细胞能量压力。相比于单一刺激如渗透压或高温,多种环境因子的相互作用对细胞能量状态的影响更为复杂,高温驯化后的机体应对降水的细胞能量代谢响应降低了可能是由于热驯化对于机体的影响,帽贝在高温下的相关能量基因的高表达提供了有效的生理抵抗从而来应对随后的渗透压压力。另一种可能是由于开始的高温驯化已经接近它们的耐受上限,以至于在面对渗透压压力时缺少生理调节能力。如果面对更高的温度的刺激,渗透压压力必然会造成机体能量的缺乏状态。同时本研究也强调了随机雨水事件对于细胞压力以及代谢调节的不利影响,证明能量代谢对机体在面临多重环境因子胁迫下至关重要的作用。
本文该研究结果于2015年12月发表于英国生态学会旗下期刊《Functional Ecology》(IF=4.828,五年影响因子5.278),ISI Journal Citation Reports® Ranking:2014: 15/144 (Ecology)。(Yunwei Dong*, Shu Zhang, 2015. Ecological relevance of energy metabolism: transcriptional responses in energy sensing and expenditure to thermal and osmotic stresses in an intertidal limpet.Functional ecology,DOI:10.1111/1365-2435.12625.)
论文链接:http://onlinelibrary.wiley.com/doi/10.1111/1365-2435.12625/full
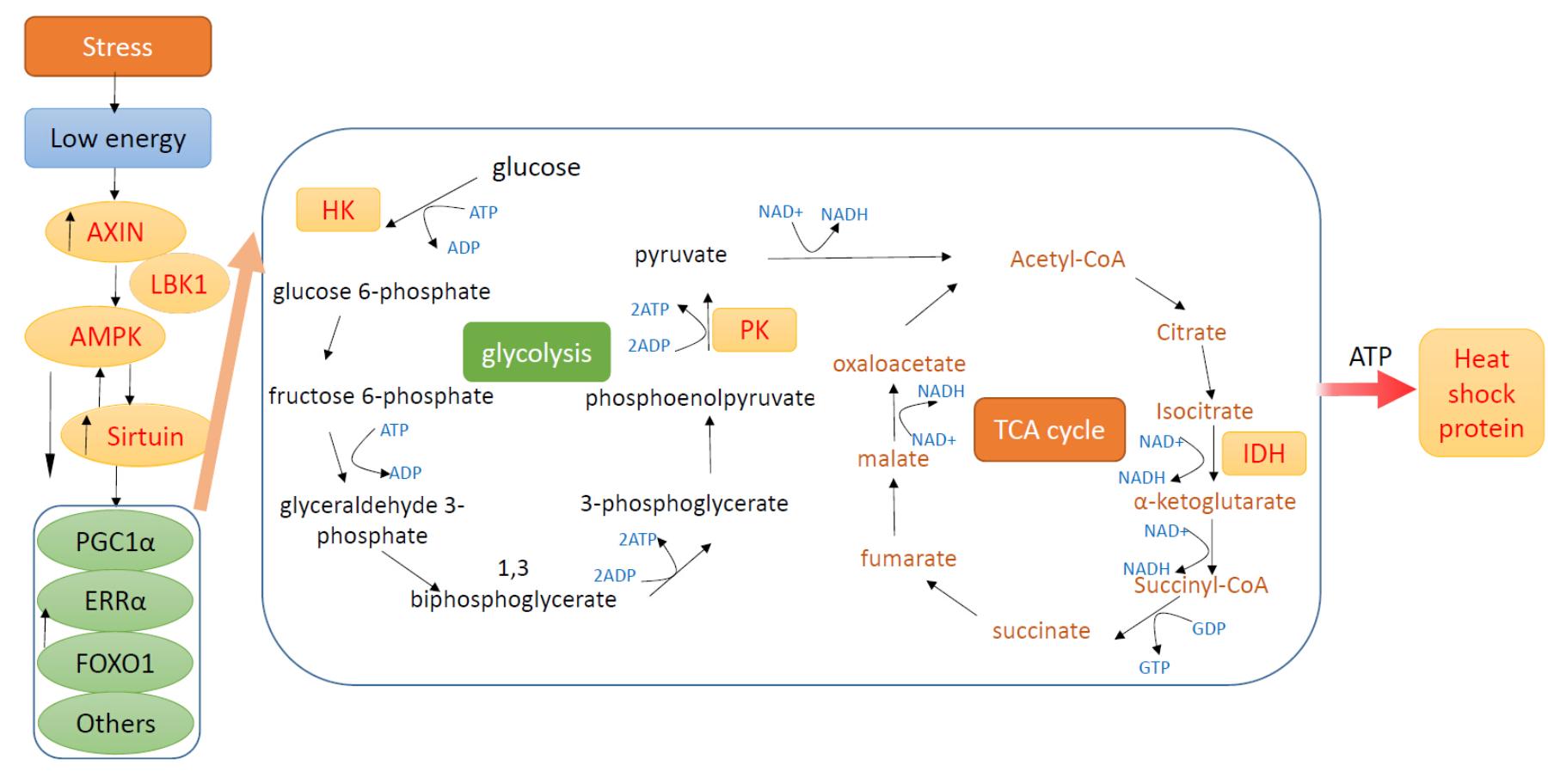
Figure 1 Scheme showing the action of metabolic sensors, enzymes involving glycolysis and the tricarboxylic acid cycle (TCA cycle), and heat shock proteins. In low cellular energy status (high AMP/ATP ratio), AMP can induce the upregulation of AXIN, AMP-activated Kinase (AMPK) and Sirtuins (SIRT). AXIN plays an essential role for AMPK activation by orchestrating AMPK and Serine-threonine liver kinase B1 (LKB1) (Zhang et al. 2013).AMPK and SIRT can activate each other. The impact of AMPK and SIRT1 on peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), estrogen related receptor α (ERRα), forkhead box O (FOXO) and other transcriptional regulators will then affect carbohydrate and lipid metabolism to produce ATP for stress responses (Cantó et al. 2009).
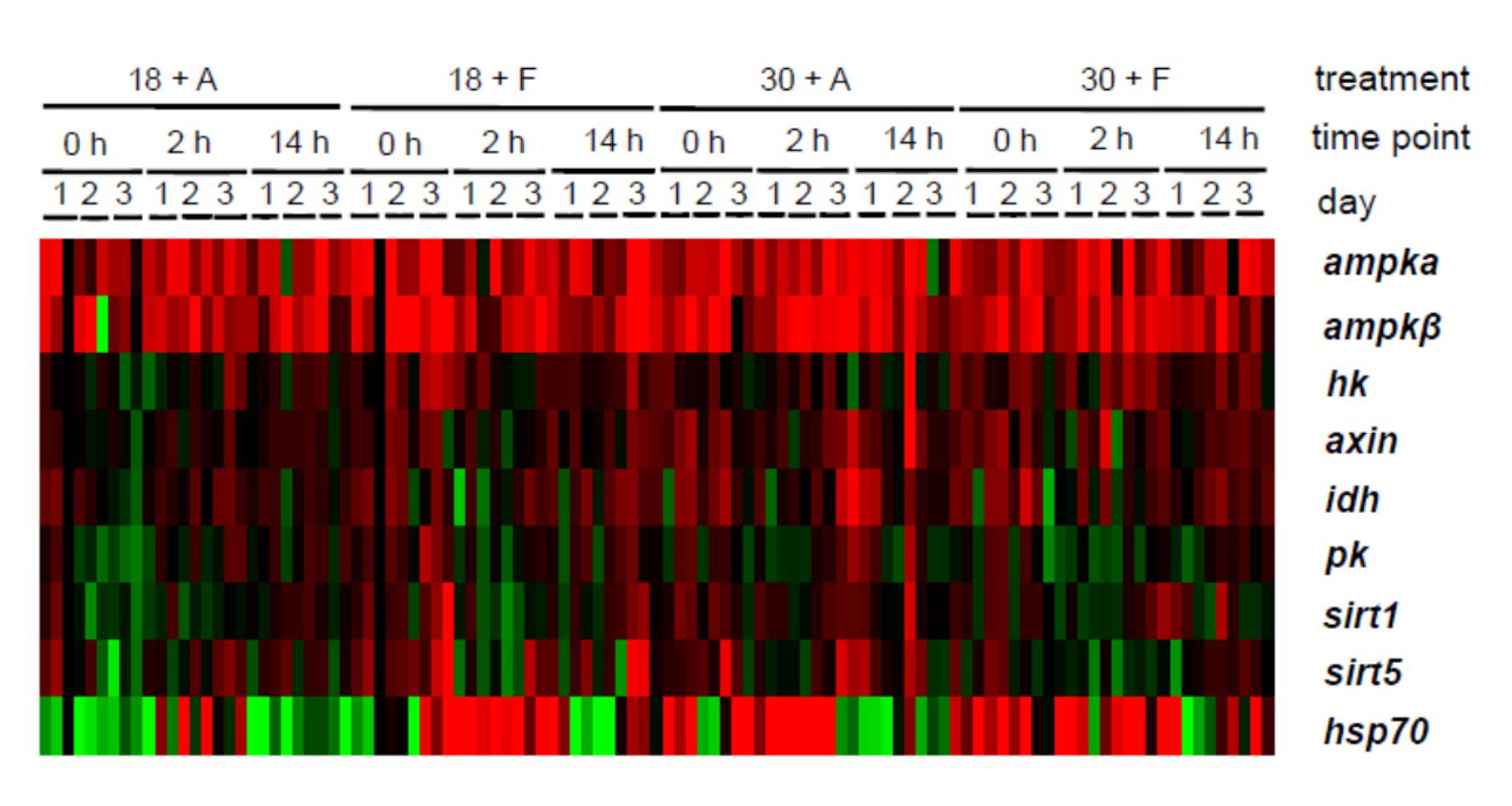
Figure 2 Gene expression of limpets in different treatments and time points. Limpets were acclimated at 18°C and 30°C. After acclimation, limpets were aerially exposed or freshwater sprayed for 2 h for three consecutive days. On each day, three limpets (n = 3) in each treatments were randomly sampled at 16:00 (before aerial exposure/freshwater spray, 0 h), 18:00 (2h after aerial exposure/freshwater spray) and next 08:00 (14 h recovery from aerial exposure/freshwater spray) for measuring gene expressions. The color scale bar indicates log-transformed data, with green indicating downregulation, red indicating upregulation and black indicating no change compared to the median of the control samples.
其他相关阅读:
1. Dong, Y.W., Han, G.D. & Huang, X.W. (2014) Stress modulation of cellular metabolic sensors: interaction of stress from temperature and rainfall on the intertidal limpet Cellana toreuma.Molecular Ecology, 23, 4541-4554.
2. Han, G.D., Zhang, S., Marshall, D.J., Ke, C.H. & Dong, Y.W. (2013) Metabolic energy sensors (AMPK and SIRT1), protein carbonylation and cardiac failure as biomarkers of thermal stress in an intertidal limpet: linking energetic allocation with environmental temperature during aerial emersion. Journal of Experimental Biology, 216, 3273-3282.